IMARC Group’s report titled “Generic Injectables Manufacturing Plant Project Report 2024: Industry Trends, Plant Setup, Machinery, Raw Materials, Investment Opportunities, Cost and Revenue” provides a comprehensive guide for establishing a generic injectables manufacturing plant. The report covers various aspects, ranging from a broad market overview to intricate details like unit operations, raw material and utility requirements, infrastructure necessities, machinery requirements, manpower needs, packaging and transportation requirements, and more.
In addition to the operational aspects, the report also provides in-depth insights into generic injectables manufacturing process, project economics, encompassing vital aspects such as capital investments, project funding, operating expenses, income and expenditure projections, fixed and variable costs, direct and indirect expenses, expected ROI, net present value (NPV), profit and loss account, and thorough financial analysis, among other crucial metrics. With this comprehensive roadmap, entrepreneurs and stakeholders can make informed decisions and venture into a successful generic injectables manufacturing unit.
Customization Available:
- Plant Location
- Plant Capacity
- Machinery- Automatic/ Semi-automatic/ Manual
- List of Machinery Provider
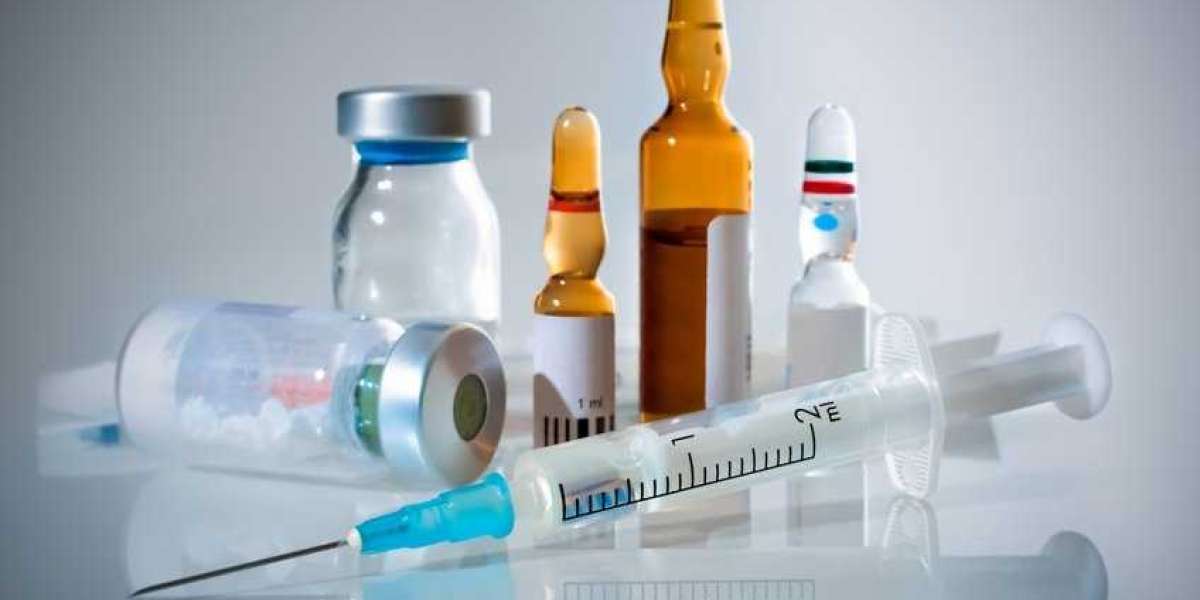
Generic injectables are pharmaceutical formulations administered via injection that are bioequivalent to brand-name drugs but produced by different manufacturers. These medications are often used in hospitals and healthcare settings for treatments ranging from anesthesia and chemotherapy to antibiotics and vaccines. Unlike oral medications, injectables bypass the digestive system, allowing for rapid and precise delivery of the active ingredient. The production of generic injectables is complex due to stringent regulatory requirements for sterility, stability, and bioequivalence, which ensures they are as safe and effective as their branded counterparts. As patents for many biologics and complex injectables expire, the market for generic injectables is expanding, offering cost-effective alternatives and driving competition.
Request For a Sample Report: https://www.imarcgroup.com/generic-injectables-manufacturing-plant-project-report/requestsample
Market trends in the generic injectables sector indicate significant growth, propelled by increasing demand for affordable healthcare solutions and an aging global population with rising chronic disease prevalence. The COVID-19 pandemic also underscored the need for reliable injectable medications, further boosting this market segment. Key factors influencing the market include regulatory approvals, technological advancements in manufacturing processes, and the entry of new players. Companies are investing in developing complex formulations and biosimilars, a subset of generic injectables derived from biological products, to capitalize on high-value opportunities. Additionally, strategic partnerships and acquisitions are common as firms seek to expand their product portfolios and global reach. The generic injectables market is poised for robust growth, driven by a blend of innovation, regulatory support, and increasing healthcare expenditure.
Key Insights Covered the Generic Injectables Plant Report
Market Coverage:
- Market Trends
- Market Breakup by Segment
- Market Breakup by Region
- Price Analysis
- Impact of COVID-19
- Market Forecast
Key Aspects Required for Setting Up a Generic Injectables Plant
Detailed Process Flow:
- Product Overview
- Unit Operations Involved
- Mass Balance and Raw Material Requirements
- Quality Assurance Criteria
- Technical Tests
Project Details, Requirements and Costs Involved:
- Land, Location and Site Development
- Plant Layout
- Machinery Requirements and Costs
- Raw Material Requirements and Costs
- Packaging Requirements and Costs
- Transportation Requirements and Costs
- Utility Requirements and Costs
- Human Resource Requirements and Costs
Project Economics:
- Capital Investments
- Operating Costs
- Expenditure Projections
- Revenue Projections
- Taxation and Depreciation
- Profit Projections
- Financial Analysis
Key Questions Addressed in This Report:
- How has the generic injectables market performed so far and how will it perform in the coming years?
- What is the market segmentation of the global generic injectables market?
- What is the regional breakup of the global generic injectables market?
- What are the price trends of various feedstocks in the generic injectables industry?
- What is the structure of the generic injectables industry and who are the key players?
- What are the various unit operations involved in a generic injectables manufacturing plant?
- What is the total size of land required for setting up a generic injectables manufacturing plant?
- What is the layout of a generic injectables manufacturing plant?
- What are the machinery requirements for setting up a generic injectables manufacturing plant?
- What are the raw material requirements for setting up a generic injectables manufacturing plant?
- What are the packaging requirements for setting up a generic injectables manufacturing plant?
- What are the transportation requirements for setting up a generic injectables manufacturing plant?
- What are the utility requirements for setting up a generic injectables manufacturing plant?
- What are the human resource requirements for setting up a generic injectables manufacturing plant?
- What are the infrastructure costs for setting up a generic injectables manufacturing plant?
- What are the capital costs for setting up a generic injectables manufacturing plant?
- What are the operating costs for setting up a generic injectables manufacturing plant?
- What should be the pricing mechanism of the final product?
- What will be the income and expenditures for a generic injectables manufacturing plant?
- What is the time required to break even?
- What are the profit projections for setting up a generic injectables manufacturing plant?
- What are the key success and risk factors in the generic injectables industry?
- What are the key regulatory procedures and requirements for setting up a generic injectables manufacturing plant?
- What are the key certifications required for setting up a generic injectables manufacturing plant?
About Us
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC Group’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145