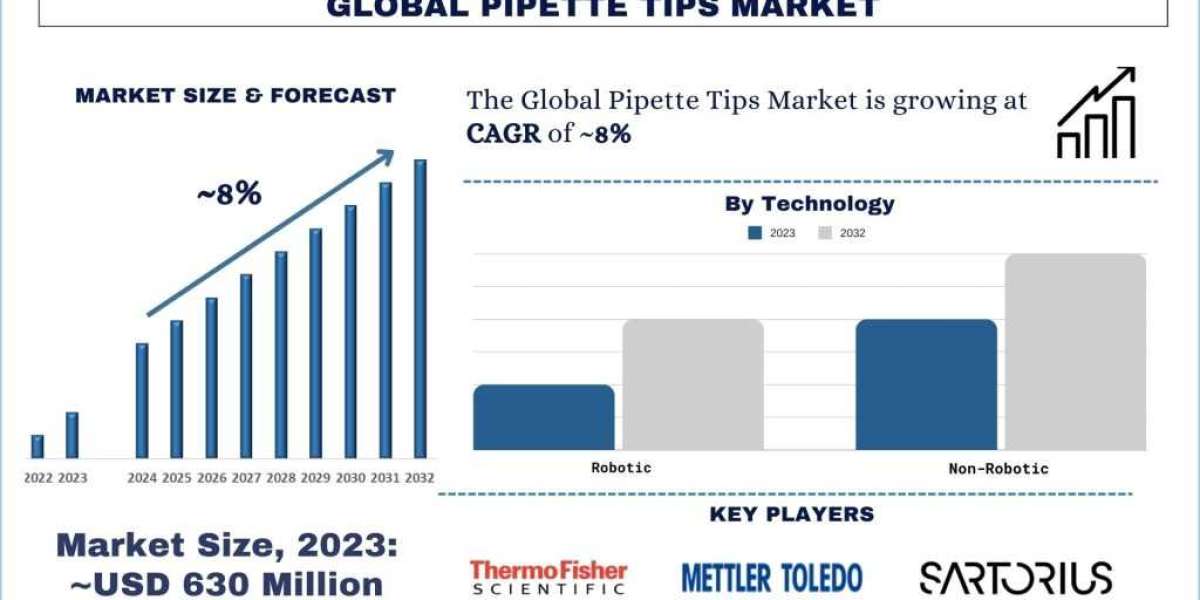
According to the UnivDatos Market Insights analysis, the high incidence of chronic diseases boosts the need for diagnostic tests, and increasing pipette tip usage will drive the scenario of the global pipette tips as per their “Global Pipette Tips Market” report, the market was valued at USD ~630 million in 2023, growing at a CAGR of ~8% during the forecast period from 2024 – 2032.
The global pipette tips market is experiencing rapid growth and transformation, driven by technological advancements, increased demand for precise laboratory instruments, and the ongoing impact of global health crises. Pipette tips, essential tools for accurate liquid handling, are indispensable in various scientific, clinical, and industrial applications.
Global Demand for Pipette Tips
The use of pipette tips is directly proportional to the growth of biotechnology and the dealing with pharmaceutical businesses. Scientists reported increasing demand for liquid handling due to high-throughput screening, drug discovery and development, and genomic research. This need was accentuated by the continuing COVID-19 pandemic that required massive amounts of diagnostic testing, vaccines, and associated research that were critical for efficient pipette tip usage. Lastly, the shift to more focused diagnoses due to molecular medicine and individual approaches in treatment also contributes to the continuous demand for these crucial lab products.
Expansion of Point-of-Care Testing
Point-of-care testing (POCT) is gaining traction in healthcare settings due to its convenience and rapid turnaround times. POCT devices often require precise liquid handling, and pipette tips are integral to these processes. The expansion of POCT for infectious diseases, chronic conditions, and emergency diagnostics is driving demand for pipette tips designed for portable and easy-to-use devices.
Digitalization and Smart Laboratories
The digital transformation of laboratories is another trend shaping the pipette tips market. Smart laboratories equipped with interconnected devices and data analytics capabilities are enhancing workflow efficiency and data management. Pipette tips integrated with digital tracking systems enable real-time monitoring of usage, inventory levels, and performance.
These smart solutions help laboratories optimize resource utilization, reduce waste, and ensure compliance with regulatory requirements. As digitalization continues to advance, the integration of smart pipette tips with laboratory information management systems (LIMS) and other digital platforms will become increasingly prevalent.
Access sample report (including graphs, charts, and figures): https://univdatos.com/get-a-free-sample-form-php/?product_id=61501
Recent Developments/Awareness Programs:- Several key players and governments are rapidly adopting strategic alliances, such as partnerships, or awareness programs for the treatment:-
- Eppendorf in its 2021 annual report stated that the company has witnessed an increase in the sales of its robotic pipetting systems which has doubled from 2020. This indicates that the demand for robotic pipettes is also likely to increase.
- In Dec. 2023, Eppendorf and Neste forged a strategic partnership to develop a new line of renewable lab plastics. The partnership aims to reduce the use of fossil oil in lab plastics by using Neste RE, a feedstock produced from 100% renewable raw materials
Regulatory Landscape
Regulations made by the government of the U. S determine how pipette tips should be manufactured, the channel of distribution as well as their usage to ensure that only quality products that meet the regulatory standards are used. The FDA categorizes pipette tips as Class I medical devices because they are considered low risk to patients and lab personnel whereas the tips that pipettes are made for a particular use are classified into certain classes. The FDA requirements such as GMP give a clearance or approval to the manufacturers who are willing to market pipette tips in the United States.
Apart from federal regulations some states or municipalities, for example, may have their standards regarding lab practices and the use of consumables, including pipette tips. This way the pipette tips are abiding by the industry norms set by the CLSI and the ISO also guarantees that they have met certain quality and performance status.
Conclusion
The global pipette tips market is undergoing significant changes, driven by technological innovations, sustainability efforts, and evolving laboratory needs. Automation and robotics, eco-friendly solutions, and advancements in tip design are shaping the future of the market. However, challenges such as supply chain disruptions, quality control, and cost management must be addressed to ensure sustained growth.
Contact Us:
UnivDatos Market Insights
Email - contact@univdatos.com
Contact Number - +1 9782263411
Website -www.univdatos.com