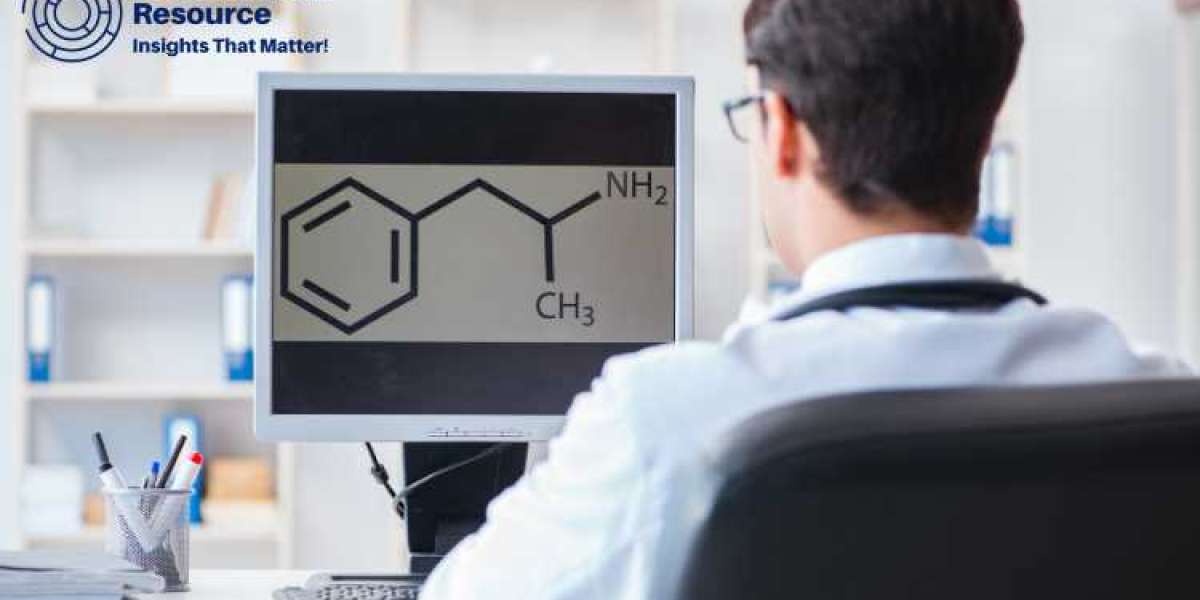
Methylamine, a simple amine derived from ammonia, is a crucial compound widely used in various industries such as pharmaceuticals, agrochemicals, and chemical synthesis. The production process of methylamine involves several stages that ensure the synthesis of high-quality and pure methylamine. Understanding the production process is essential for businesses looking to optimize their manufacturing and reduce production costs.
Manufacturing Report and Process
The manufacturing of methylamine typically follows two primary processes: the reaction of ammonia with methanol and the catalytic reaction of methanol with an amine. Each method has its own advantages and applications based on the desired end use and scale of production.
Request For Sample: https://www.procurementresource.com/production-cost-report-store/methylamine/request-sample
1. Ammonia and Methanol Reaction
In this process, methylamine is produced by the reaction of ammonia (NH3) with methanol (CH3OH) in the presence of a catalyst.
Step-by-Step Process:
- Preparation of Reactants: High-purity ammonia and methanol are prepared and stored in appropriate conditions to prevent contamination.
- Catalyst Preparation: Catalysts such as alumina-supported metal catalysts are prepared and activated to enhance the reaction rate and selectivity.
- Reaction Stage: The reactants are fed into a reactor where the catalytic reaction takes place at elevated temperatures (250-450°C) and pressures (10-20 atm). The presence of a catalyst helps in breaking down the methanol molecule and facilitating the formation of methylamine.
- Separation and Purification: The product mixture containing methylamine, water, and unreacted reactants is subjected to separation processes such as distillation. Methylamine is separated from water and any residual ammonia or methanol to obtain a pure product.
- Storage and Handling: The purified methylamine is then stored in specialized containers to prevent degradation or contamination before being transported to its end-use applications.
2. Catalytic Reaction of Methanol with Amine
Another method involves the catalytic reaction of methanol with an amine such as ammonia. This method is often used when high purity and yield are required.
Step-by-Step Process:
- Reactant Preparation: High-purity methanol and ammonia are prepared and introduced into the reaction system.
- Catalyst Activation: Catalysts such as zeolites or other metal-based catalysts are activated and loaded into the reactor.
- Reaction Phase: The reaction occurs in a continuous or batch reactor under controlled temperature and pressure conditions. The catalyst aids in the formation of methylamine from methanol and ammonia.
- Product Recovery: The reaction mixture is then processed to separate methylamine from the by-products and unreacted reactants. Techniques like distillation, absorption, and adsorption are used to achieve this separation.
- Purification and Storage: The recovered methylamine is purified to remove any impurities and then stored in appropriate conditions to maintain its quality until it is needed for further use.
Raw Material Costs
Raw material costs play a significant role in determining the overall production cost of methylamine. The main raw materials involved in the production process are ammonia and methanol, and their market prices can significantly impact the cost-efficiency of the production process.
Ammonia
Ammonia is a critical raw material in the methylamine production process. It is commonly produced using the Haber-Bosch process, which involves the reaction of nitrogen (N2) from the air with hydrogen (H2) derived from natural gas. The cost of ammonia is influenced by several factors, including the price of natural gas, energy costs, and production scale.
Factors Affecting Ammonia Costs:
- Natural Gas Prices: Since natural gas is a primary feedstock for ammonia production, fluctuations in natural gas prices can directly impact ammonia costs.
- Energy Costs: The Haber-Bosch process is energy-intensive, and variations in energy prices can affect the overall cost of ammonia production.
- Supply and Demand: Market dynamics, including supply and demand for ammonia, can lead to price variations. Seasonal demand for fertilizers, for instance, can drive up ammonia prices.
Methanol
Methanol is another crucial raw material used in the production of methylamine. Methanol is typically produced from natural gas through a process known as steam methane reforming (SMR), followed by methanol synthesis.
Factors Affecting Methanol Costs:
- Natural Gas Prices: Similar to ammonia, the cost of methanol is heavily influenced by natural gas prices, as natural gas is the primary feedstock.
- Production Technology: Advances in production technology and efficiency can impact methanol production costs. Technologies that improve yield and reduce energy consumption can lower costs.
- Market Demand: Methanol demand in various sectors, including fuel, chemicals, and plastics, can affect its market price. High demand in these sectors can lead to increased prices.
Cost Analysis
To understand the production cost structure of methylamine, it is essential to analyze the contributions of raw material costs, energy costs, labor costs, and overheads.
- Raw Material Costs: These constitute a significant portion of the total production cost. The combined cost of ammonia and methanol, along with any additional reactants or catalysts, forms the bulk of raw material expenses.
- Energy Costs: The energy required for heating, cooling, and maintaining the reaction conditions also contributes to the overall cost. Energy efficiency measures can help in reducing these costs.
- Labor Costs: Skilled labor is needed to operate and maintain the production facilities. Labor costs vary based on the region and the complexity of the production process.
- Overhead Costs: These include costs related to facility maintenance, safety measures, environmental compliance, and other administrative expenses.
Optimizing Production Costs
To optimize production costs, manufacturers can focus on several strategies:
- Improving Yield: Enhancing the yield of the methylamine production process can reduce raw material consumption and waste generation.
- Energy Efficiency: Implementing energy-efficient technologies and practices can significantly lower energy costs.
- Raw Material Sourcing: Securing long-term contracts for raw material supply at competitive prices can help stabilize costs.
- Process Optimization: Continuous monitoring and optimization of the production process can lead to cost savings through improved efficiency and reduced downtime.
Conclusion
The production cost of methylamine is influenced by various factors, including raw material costs, energy expenses, labor, and overheads. Understanding the production process and the associated costs is essential for manufacturers to optimize their operations and remain competitive in the market. By focusing on yield improvement, energy efficiency, and strategic raw material sourcing, businesses can achieve cost-effective methylamine production, ensuring high-quality products for their end-use applications.