Market Overview
The Laboratory Proficiency Testing Market is experiencing significant growth, driven by the increasing focus on quality assurance in clinical and diagnostic laboratories, regulatory compliance requirements, and advancements in analytical testing. Proficiency testing (PT) is crucial in ensuring the accuracy, reliability, and standardization of laboratory testing procedures across various industries, including healthcare, environmental testing, pharmaceuticals, and food safety.
With the rise in accreditation requirements for diagnostic laboratories, the adoption of external quality assessment (EQA) programs has surged. Additionally, the growing demand for molecular diagnostics and microbiology testing has further expanded the scope of proficiency testing in modern laboratories.
Market Size and Share
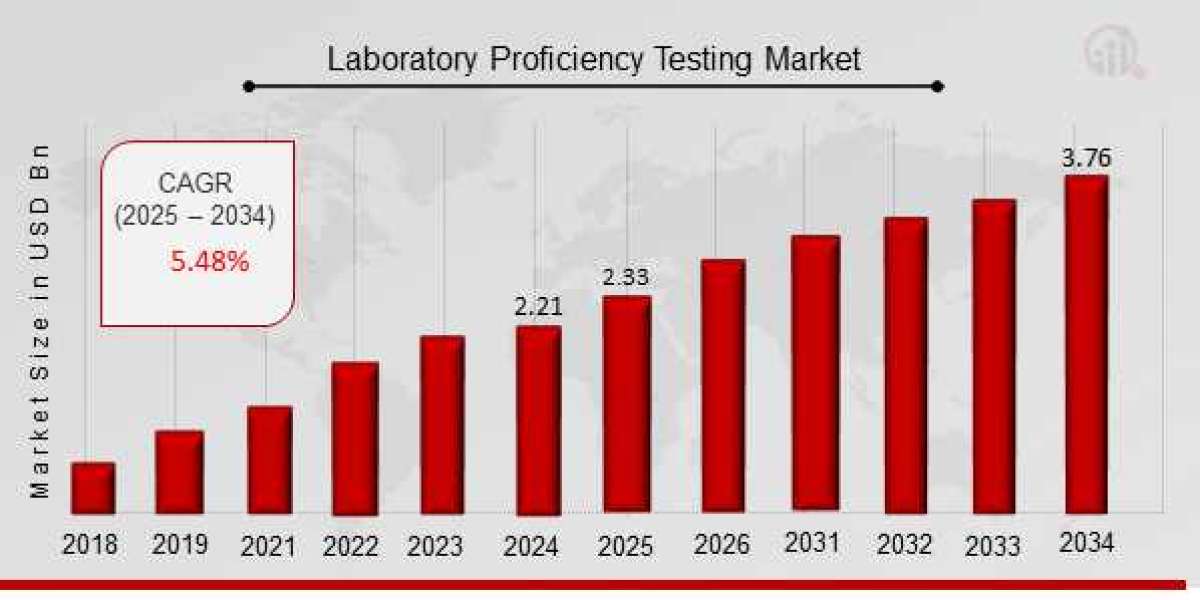
The Laboratory Proficiency Testing Market Size was estimated at 2.21 (USD Billion) in 2024. The Laboratory Proficiency Testing Market Industry is expected to grow from 2.33 (USD Billion) in 2025 to 3.76 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 5.48% during the forecast period (2025 - 2034).
North America holds the largest market share due to strict regulatory guidelines, high adoption of clinical laboratory accreditation programs, and an increasing number of reference laboratories. The Asia-Pacific region is anticipated to experience the fastest growth due to rising investments in laboratory infrastructure and growing awareness about quality assurance in diagnostic labs.
Market Drivers
- Stringent Regulatory Compliance Requirements: Government and private healthcare bodies mandate the use of proficiency testing programs to ensure laboratory accuracy.
- Growing Demand for Accurate Diagnostic Testing: With the increasing prevalence of infectious diseases, chronic conditions, and genetic disorders, the need for error-free laboratory testing has risen.
- Technological Advancements in Laboratory Automation: The integration of AI-powered laboratory testing solutions and automated analytical instruments has improved efficiency in testing.
- Rising Adoption of External Quality Assurance (EQA) Programs: Laboratories worldwide are increasingly participating in EQA schemes to benchmark their performance and maintain certification.
- Expansion of Molecular Microbiology Testing: The growing use of PCR-based diagnostics, NGS, and microbiology assays has expanded proficiency testing applications.
Challenges and Restraints
- High Costs Associated with Proficiency Testing Programs: Many laboratories, especially in developing regions, struggle with the affordability of external quality assessment services.
- Limited Awareness in Emerging Economies: Lack of proper knowledge about the benefits of quality control testing in laboratories affects market penetration.
- Challenges in Standardization of Testing Procedures: Variability in laboratory testing protocols across different regions can hinder the effectiveness of proficiency testing programs.
Market Trends
- Increased Use of Digital AI-Driven Proficiency Testing Solutions: AI and machine learning algorithms for laboratory quality control are improving proficiency testing accuracy.
- Rising Adoption of Proficiency Testing in Food Water Safety: Beyond healthcare, environmental testing laboratories are increasingly utilizing PT programs.
- Expansion of Molecular Genetic Proficiency Testing Programs: NGS-based laboratory testing assessments are gaining traction in precision medicine research.
- Integration of Cloud-Based Laboratory Quality Control Solutions: Many labs are adopting cloud-based PT data management systems for real-time performance tracking.
Regional Analysis
- North America: Dominates the market due to strong regulatory policies, high participation in CAP proficiency testing programs, and the presence of leading diagnostic companies.
- Europe: Growing demand for ISO 15189-accredited laboratories and increased government funding for EQA programs support market growth.
- Asia-Pacific: Rising awareness about laboratory accreditation and expansion of diagnostic facilities drive regional growth.
- Rest of the World: The adoption of proficiency testing services in pharmaceutical and clinical laboratories is gradually increasing.
Segmental Analysis
- By Industry:
- Clinical Diagnostics
- Pharmaceuticals Biotechnology
- Environmental Water Testing
- Food Beverage Testing
- By Technology:
- Microbiology
- Molecular Diagnostics
- Immunology
- Clinical Chemistry
- Haematology
- By End-User:
- Hospitals Clinical Laboratories
- Research Academic Institutes
- Pharmaceutical Companies
- Government Regulatory Agencies
Key Market Players
- Thermo Fisher Scientific
- College of American Pathologists
- International Accreditation Services
- Quality Control Solutions
- BD
- Roche Diagnostics
Recent Developments
- Expansion of Proficiency Testing Programs: CAP launched new molecular pathology PT programs to support genetic and infectious disease testing.
- Integration of AI in Laboratory Quality Control: Bio-Rad introduced an AI-powered laboratory proficiency testing software to streamline data analysis.
- Collaborations for Global Standardization: Thermo Fisher partnered with regulatory bodies to develop universal PT guidelines for clinical laboratories.
For more information, please visit us at marketresearchfuture.