Introduction
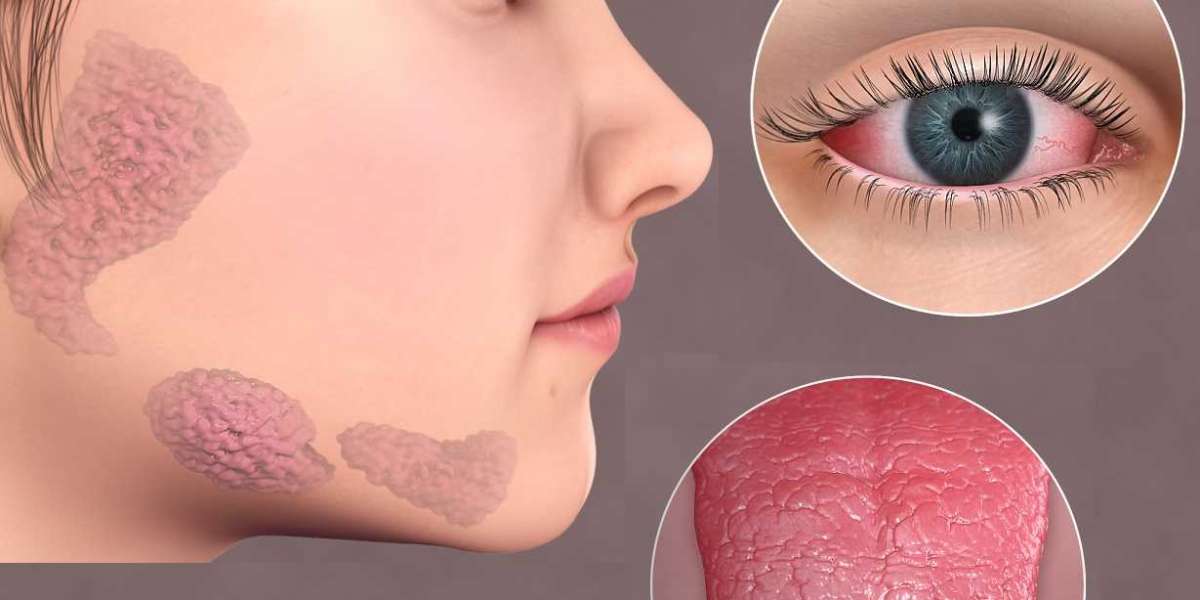
Sjögren’s Syndrome is a chronic autoimmune disorder that primarily affects the body’s moisture-producing glands, leading to severe dryness in the eyes and mouth. This debilitating condition not only causes persistent discomfort but also increases the risk of vision impairment and corneal damage. Traditional treatment options—such as artificial tears, anti-inflammatory drops, and immunosuppressive therapies—primarily address symptoms rather than the underlying causes. As the search for more definitive and restorative treatments intensifies, innovative therapies that target the root of ocular surface damage are emerging as promising alternatives.
One such breakthrough is OXERVATE, a therapy that harnesses the regenerative power of its active ingredient, cenegermin. Initially approved for the treatment of neurotrophic keratitis, OXERVATE is now being explored as a potential treatment for the ocular complications associated with Sjögren’s Syndrome. This article delves into the science behind OXERVATE’s Mechanism of Action, examines its market performance and sales trends, discusses cost considerations, and highlights ongoing clinical trials. Together, these aspects paint a comprehensive picture of how OXERVATE could shape the future of ophthalmic care for patients with Sjögren’s Syndrome.
For more in-depth insights on OXERVATE’s development and future potential, download the full report @ OXERVATE Market Report.
Understanding OXERVATE's Mechanism of Action (MOA)
At the heart of OXERVATE’s promise lies its unique mechanism of action. The therapy’s active ingredient, cenegermin, is a recombinant human nerve growth factor (rhNGF) that plays a pivotal role in maintaining the integrity of the corneal surface. By stimulating nerve regeneration and promoting the healing of corneal epithelial cells, OXERVATE’s Mechanism of Action addresses one of the key issues in Sjögren’s Syndrome—chronic dry eye and the subsequent risk of corneal damage.
In patients with Sjögren’s Syndrome, the loss of moisture not only causes discomfort but also impairs the natural repair processes of the cornea. The OXERVATE active ingredient is designed to counteract this effect by enhancing tear production and supporting the survival of corneal cells. This regenerative approach goes beyond the temporary relief provided by conventional treatments and aims to restore a more natural, long-term balance to the ocular surface. By focusing on nerve health and epithelial repair, OXERVATE offers a novel solution that could potentially mitigate the debilitating symptoms of Sjögren’s Syndrome while improving overall corneal health.
The Need for Innovative Treatments in Sjögren’s Syndrome
Current treatment modalities for Sjögren’s Syndrome-related dry eye are largely palliative in nature. Patients often rely on frequent use of artificial tears and anti-inflammatory drops to manage discomfort, but these treatments fail to address the underlying nerve dysfunction that exacerbates ocular surface disease. As a result, many individuals continue to experience persistent symptoms and progressive damage despite adherence to existing therapeutic regimens.
The limitations of these conventional approaches underscore the urgent need for innovative treatments that not only alleviate symptoms but also promote genuine tissue repair and regeneration. In this context, OXERVATE emerges as a beacon of hope. By targeting the neurodegenerative aspects of ocular disease with its regenerative active ingredient, OXERVATE may offer more sustained and effective relief. This potential to correct the underlying nerve damage is particularly significant for Sjögren’s Syndrome, where chronic dryness and corneal injury are major concerns. With its distinct mechanism of action, OXERVATE could pave the way for a new era in ophthalmic care—one that moves from mere symptomatic management to true restorative therapy.
For more detailed insights and the latest updates on OXERVATE, visit the OXERVATE Market update.
OXERVATE Sales and Market Performance
Since its initial FDA approval for neurotrophic keratitis, OXERVATE has not only garnered attention for its innovative therapeutic approach but also demonstrated impressive market performance. The robust OXERVATE sales figures are a testament to the confidence that both clinicians and patients have in the treatment’s efficacy. These strong sales numbers have been driven largely by the growing demand for advanced ophthalmic therapies that provide long-term benefits.
The story of OXERVATE sales is one of consistent growth and market expansion. As healthcare providers continue to witness the tangible benefits of a treatment that enhances nerve regeneration and promotes epithelial healing, the adoption of OXERVATE has steadily increased. The promising results observed in its approved indication for neurotrophic keratitis have set a positive precedent, suggesting that further indications—such as its potential use in Sjögren’s Syndrome—could drive even greater market acceptance. In fact, industry experts predict that if the ongoing OXERVATE Clinical Trials yield favorable outcomes, the already strong OXERVATE sales could surge further, cementing its status as a revolutionary therapy in ophthalmology.
Moreover, the continued interest in OXERVATE is expected to bolster its presence in global markets. With healthcare systems increasingly prioritizing treatments that offer regenerative and long-lasting benefits, OXERVATE’s sales performance provides a clear signal of its potential to transform ophthalmic care. The current momentum in OXERVATE sales not only reflects its efficacy but also highlights the growing appetite for therapies that move beyond conventional symptomatic relief. As the treatment continues to gain traction, the dual focus on scientific innovation and market performance will be key drivers of its future success.
For further insights and detailed research on this breakthrough treatment, visit OXERVATE insights.
Cost Considerations: OXERVATE Price and Accessibility
One of the primary challenges in the widespread adoption of advanced biologic therapies like OXERVATE is the associated cost. As a treatment that relies on a sophisticated manufacturing process for its recombinant active ingredient, OXERVATE comes with a high price tag. In the United States, for example, an eight-week treatment course has been reported to cost approximately $96,000. This substantial cost can present a significant barrier to access for many patients, particularly when compared to traditional therapies that offer only symptomatic relief.
The high cost of OXERVATE is a reflection of both the complexity involved in producing the OXERVATE active ingredient and the rigorous regulatory standards that govern biologic therapies. Despite these challenges, the potential benefits of OXERVATE—especially its ability to restore corneal health and nerve function—make it a compelling option for patients with severe ocular surface disease. In the case of Sjögren’s Syndrome, where conventional treatments have fallen short, the investment in a regenerative therapy may well be justified by the prospect of long-term improvement in quality of life.
To improve accessibility, it is crucial for healthcare policymakers, insurance companies, and pharmaceutical companies to collaborate on strategies that can help offset these costs. Expanded insurance coverage, patient assistance programs, and innovative pricing models could play a vital role in ensuring that more patients benefit from OXERVATE. Ultimately, addressing these cost considerations will be essential for the broader adoption of OXERVATE, especially as it moves closer to potential regulatory approvals for additional indications such as Sjögren’s Syndrome.
For additional insights on OXERVATE’s transformative potential, please download the full OXERVATE report.
Clinical Trials and Future Prospects
The exploration of OXERVATE as a treatment option for Sjögren’s Syndrome is still in its early stages, with clinical trials underway to assess its safety, efficacy, and long-term benefits in this new context. These OXERVATE Clinical Trials are critical in determining whether the regenerative properties observed in neurotrophic keratitis can be successfully translated to alleviate the chronic dry eye and corneal damage seen in Sjögren’s Syndrome patients.
Initial research indicates that the OXERVATE active ingredient may offer significant advantages by promoting nerve regeneration and epithelial healing—key factors that are compromised in Sjögren’s Syndrome. If these clinical trials confirm its effectiveness, the implications could be profound, paving the way for new OXERVATE Approvals that extend beyond its current indication. Such regulatory milestones would not only validate the treatment’s novel mechanism but also potentially broaden its market reach, further enhancing OXERVATE sales.
The future prospects for OXERVATE in the realm of ophthalmic care appear promising. As the scientific community continues to investigate its potential applications, the possibility of a breakthrough in the management of Sjögren’s Syndrome becomes increasingly tangible. Positive outcomes from these clinical trials could lead to a paradigm shift in the treatment of autoimmune-related ocular surface disorders, positioning OXERVATE as a cornerstone therapy that offers both symptomatic relief and true regenerative benefits. This forward-thinking approach is precisely what the field of ophthalmology needs to address the evolving challenges posed by chronic autoimmune conditions.
For those looking to explore more about this breakthrough treatment, download the full OXERVATE Insights Report.
Conclusion
OXERVATE stands at the forefront of a potential revolution in ophthalmic care. With its innovative active ingredient and distinctive Mechanism of Action, it offers a new therapeutic avenue that goes beyond traditional symptomatic treatments for Sjögren’s Syndrome. The encouraging performance reflected in current OXERVATE sales, combined with ongoing clinical investigations, underscores its potential to redefine how ocular surface diseases are managed. While challenges such as high treatment costs and the need for further clinical validation remain, the prospect of expanded OXERVATE Approvals could mark a significant turning point for patients suffering from severe dry eye and corneal damage.
As researchers continue to explore and validate the benefits of the OXERVATE active ingredient in Sjögren’s Syndrome, the future looks increasingly promising. OXERVATE’s ability to promote nerve regeneration and epithelial healing may well usher in a new era in ophthalmic care—one that prioritizes long-term restoration and improved quality of life for patients. With strong market performance already evident through robust OXERVATE sales, the ongoing clinical trials will be key to unlocking further potential and cementing its role as a groundbreaking treatment option.
Related Reports
- Dry Eye Disease Market Insight, Epidemiology And Market Forecast Report
- Sjogren's Syndrome Market Insight, Epidemiology And Market Forecast Report
- Ocular Hypertension Market Insight, Epidemiology And Market Forecast Report
About DelveInsight
DelveInsight is a leading business Healthcare consultancy and market research firm specializing in life sciences. It assists pharmaceutical companies by offering comprehensive, end-to-end solutions to improve their performance. Access all our healthcare and pharmaceutical market Competitive Intelligence Solutions.