In the realm of chemical synthesis and bioconjugation, 1-Ethyl-3-(3-Dimethylaminopropyl)Carbodiimide Hydrochloride, commonly known as EDC HCL, stands out as a versatile and essential reagent. Whether you are a researcher, chemist, or involved in the pharmaceutical industry, understanding the properties, uses, and applications of EDC HCL can significantly enhance your work. This comprehensive guide provides an in-depth look at EDC HCL, including its composition, uses, working mechanism, handling precautions, etc.
What is 1-Ethyl-3-(3-Dimethylaminopropyl)Carbodiimide Hydrochloride?
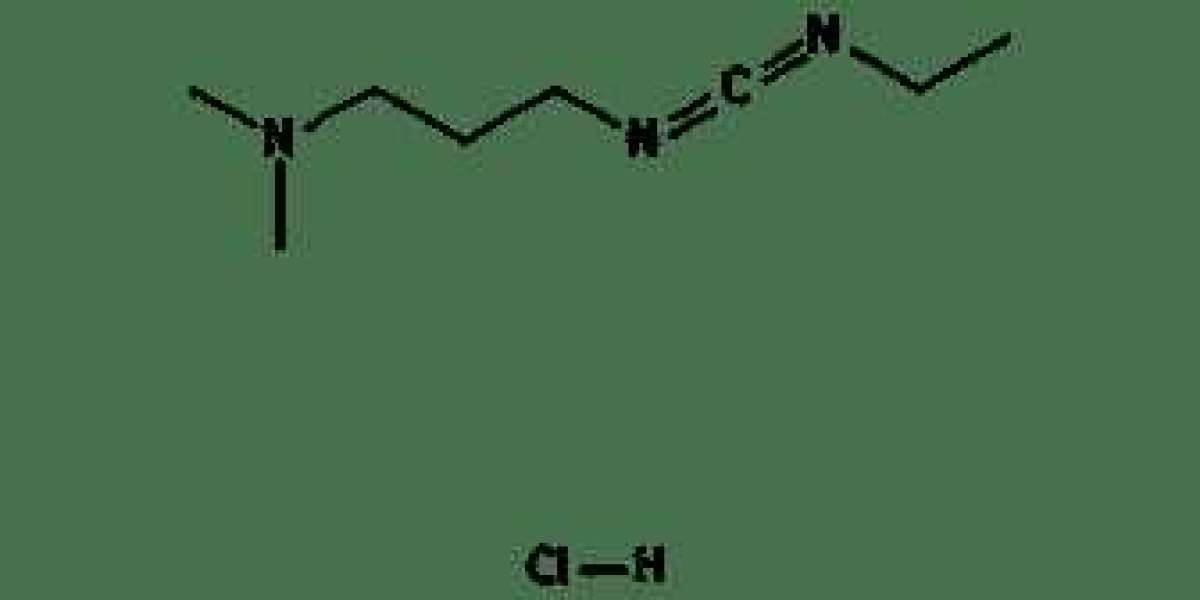
1-Ethyl-3-(3-Dimethylaminopropyl)Carbodiimide Hydrochloride (EDC HCL) is a water-soluble carbodiimide reagent widely used in peptide synthesis and the conjugation of proteins and nucleic acids. It facilitates the formation of amide bonds between carboxyl groups and primary amines, making it a critical component in biochemical research and molecular biology.
About EDC HCL
- Chemical Formula: C8H17N3·HCl
- Molecular Weight: 191.70 g/mol
- Appearance: White to off-white crystalline powder
- Solubility: Soluble in water and various organic solvents
- Case No: 25952-53-8
Manufacturer
EDC HCL is produced by numerous chemical manufacturers and suppliers specializing in laboratory reagents and fine chemicals. Leading suppliers ensure high purity and stringent quality control to provide reliable and consistent performance in research and industrial applications.
Most Selling Products:-
What Are the Uses of EDC HCL?
EDC HCL is used primarily for:
- Peptide Synthesis: EDC HCL is commonly used to activate carboxyl groups in amino acids, facilitating the formation of peptide bonds during solid-phase peptide synthesis.
- Protein Conjugation: It enables the conjugation of proteins and peptides by forming stable amide bonds, essential in antibody-drug conjugates and bioconjugation studies.
- Nucleic Acid Research: EDC HCL is utilized in cross-linking nucleic acids and proteins, aiding in developing nucleic acid-based assays and diagnostics.
- Carbodiimide Chemistry: It is employed in various organic synthesis reactions, particularly in the formation of esters, amides, and anhydrides.
How EDC HCL Works
EDC HCL works by activating carboxyl groups (COOH) to form an O-acylisourea intermediate, which is highly reactive. This intermediate can then react with primary amines (NH2) to form stable amide bonds. The overall reaction involves the following steps:
- Activation: EDC HCL reacts with the carboxyl group to form the O-acylisourea intermediate.
- Coupling: The intermediate reacts with a primary amine to form the desired amide bond.
- Byproduct Formation: The reaction produces a urea byproduct, which can be removed by subsequent washing or purification steps.
Handling and Storage
Proper handling and storage of EDC HCL are crucial to maintain its stability and effectiveness:
- Storage: Store EDC HCL in a cool, dry place, preferably between 2-8°C. Keep it tightly sealed and protected from moisture and light.
- Handling: Use appropriate personal protective equipment (PPE), gloves, and safety goggles when handling EDC HCL. Work in a well-ventilated area or under a fume hood to avoid inhalation of dust.
Precautions
When using EDC HCL, consider the following safety precautions:
- Health Hazards: EDC HCL can irritate the skin, eyes, and respiratory system. Avoid direct contact and inhalation of dust.
- Chemical Stability: Ensure that EDC HCL is stored properly to prevent degradation. Exposure to moisture can lead to hydrolysis, reducing its efficacy.
- Environmental Safety: Dispose of EDC HCL and its byproducts according to local regulations and guidelines for hazardous waste.
Side Effects and Toxicity
- Acute Exposure: It may cause irritation to the skin, eyes, and mucous membranes, and inhalation can lead to respiratory discomfort.
- Chronic Exposure: Prolonged or repeated exposure may cause sensitization and allergic reactions in some individuals.
Applications in Research and Industry
- Bioconjugation: EDC HCL is widely used to prepare bioconjugates for diagnostic and therapeutic applications.
- Material Science: Utilized in functionalizing surfaces and materials for improved properties and performance.
- Pharmaceutical Development: Plays a critical role in synthesizing drug candidates and active pharmaceutical ingredients (APIs).
FAQ
1. Can EDC HCL be used in aqueous solutions?
- Yes, EDC HCL is water-soluble and can be used in aqueous solutions for various biochemical applications.
2. How should I dispose of EDC HCL?
- Dispose of EDC HCL according to local hazardous waste disposal regulations. Consult your institution's safety guidelines for proper disposal methods.
3. What is the shelf life of EDC HCL?
- EDC HCL has a shelf life of 1-2 years when stored properly. Check the manufacturer's specifications for exact details.
4. Can EDC HCL be used with other coupling reagents?
- Yes, EDC HCL can be combined with other coupling reagents, such as N-hydroxysuccinimide (NHS), to improve the coupling efficiency and stability of the amide bond.
5. Is EDC HCL compatible with all solvents?
- EDC HCL is soluble in water and various organic solvents like dimethyl sulfoxide (DMSO) and dimethylformamide (DMF). However, its reactivity may vary depending on the solvent used.
6. What should I do if EDC HCL comes into contact with my skin?
- Rinse the affected area with plenty of water and seek medical attention if irritation persists.
Conclusion
1-Ethyl-3-(3-Dimethylaminopropyl)Carbodiimide Hydrochloride (EDC HCL) is an indispensable reagent in the fields of peptide synthesis, protein conjugation, and nucleic acid research. Its ability to facilitate amide bond formation makes it a valuable tool for scientists and researchers. By understanding its properties, uses, and handling precautions, you can effectively and safely incorporate EDC HCL into your laboratory practices.